An update on COVID-19 research
Several publications over the past week have started to focus on the differences observed in COVID-19 patients. While it is widely reported that COVID-19 has “specific” populations that it affects more severely than others (men, elderly, those with other health morbidities) it isn’t exactly known why these differences exist.
Research groups across the world have been investigating these disparities. Dr. Balaji has shared research from Wang, Hansen and colleagues who suggest that the composition of cell membranes, which varies throughout a person’s life, may be responsible for increased infection and possibly disease severity. Other groups have been examining other aspects of these patients to try to determine if there are additional differences that may explain these disparities and provide diagnostic and therapeutic opportunities.
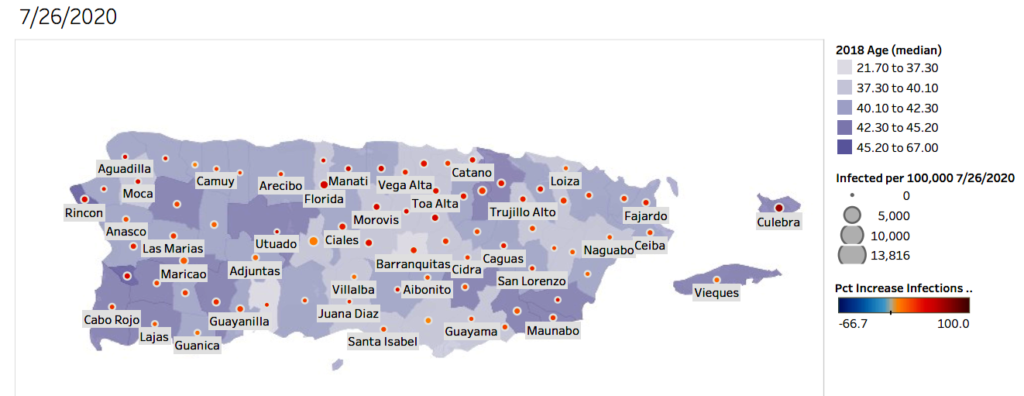
Increased cases of COVID-19 in Puerto Rico over the past two weeks (7-12-2020 through 7-26-2020). This graph presents the percent increase over the past two weeks and represents the cumulative/total number of cases. Each municipality is colored with the median age of the population. Data from JHU-CSSE.
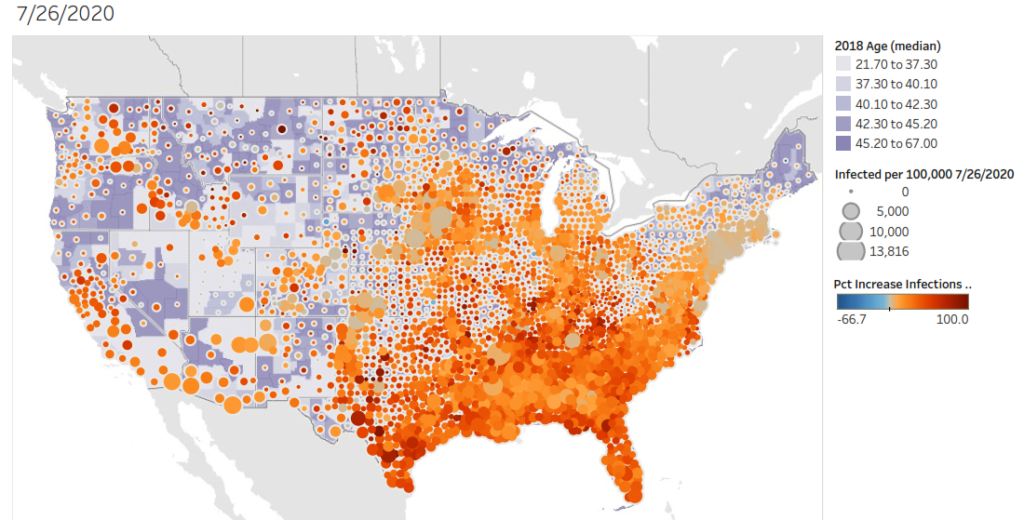
Increased cases of COVID-19 in the United States over the past two weeks (7-12-2020 through 7-26-2020). This graph presents the percent increase over the past two weeks and represents the cumulative/total number of cases. Each county is colored with the median age of the population. Data from JHU-CSSE .
COVID-19 Review1
COVID-19 is a disease caused by infection with the SARS-CoV2 virus. Entry into the cell allows the virus to replicate and infect other cells, while severely compromising cellular, tissue and organ function. Recognition of infection by the immune system mobilizes a robust response against the infection, at least initially.
In severe and critical cases of COVID-19, which have the highest risk or fatality and complications, there is mounting evidence for lymphopenia (T and B cell depletion) and for infiltration from myeloid cells. Myeloid cells are a type of immune cell involved in innate immunity and not tailored for response to viruses.
It appears that an initial/early response gives way to a late/mature response where adaptive immune cells are either ineffective or absent. The inflammation caused in the early stages of the response appears to impair expansion of T-cells and leads to the apoptosis of those cells that are present.
Identifying T-cell populations in patients with COVID-192
In a recent study published in the online pre-print bioRxiv.org Mathew and colleagues examined 125 patients and profiled them using deep immune profiling through flow-cytometry. Most of the patients examined are similar to a typical severe COVID-19 patient, being male and around 60 years of age. The patients were skewed towards African-American (68%) with cardiovascular risk factors present in 83%.
About one fifth of patients (18%) were immune suppressed for reasons not related to COVID-19. Most patients had been treated for their COVID-19 diagnosis (45% hydroxychloroquine, 31% with steroids and 29% with remdesivir). These characteristics may impact the results of the groups research and they will need to be controlled in future research.
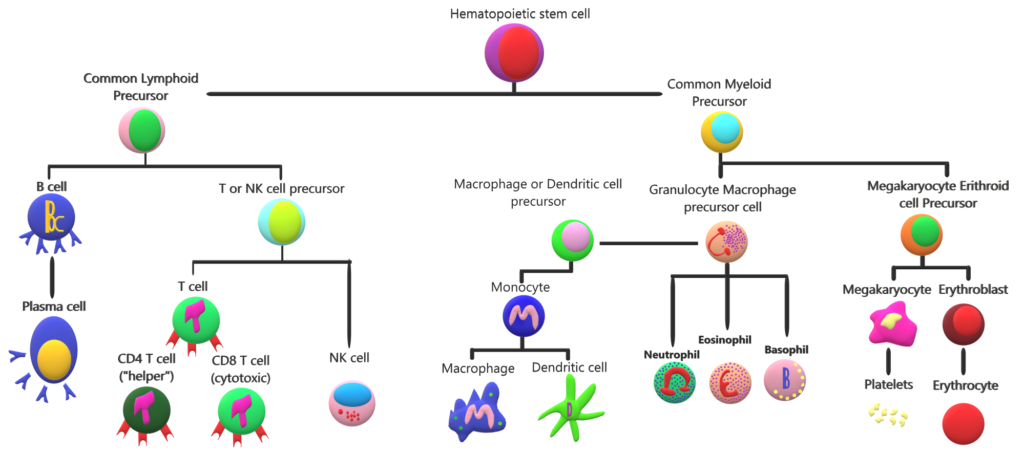
A hematopoietic cell “family tree”. Hematopoietic cells mediate systemic functions from oxygenation to damage repair and pathogen removal. In this graph we can see that T cells are related to myeloid cells. These two cell types carry out different immune functions. The presence or absence of a sub-set of cells results in the variety of responses that our immune system is capable of carrying out. In COVID-19 the absence or “exhaustion” of T cells results in a weak immune response that may lead to damage of surrounding tissue.
Most patients were enrolled nine days after symptom development. Markers of inflammation immune response, blood clotting and cell death were elevated in most patients (>75%). The inflammatory cytokine IL-6 was elevated in 88% of patients.
The purpose of the experiment was to compare patients with active COVID-19 to those that have either never had COVID-19 and a group that had recovered (control groups). The researchers found differences between these groups that suggest three distinct COVID-19 patient populations based on T cell response.
The research group confirmed the known clinical symptom of lymphopenia (B and T-cells decrease) in their COVID-19 patients. While total white blood cell counts were elevated they found that B or T cells were not increased. This is because the elevated cells belong to the myeloid type of immune cell.
The researchers identified three types of patients based on the presence of proteins and clinical outcomes. Disparities or T cell diversity may have an impact on regulation of the immune response. This finding may help identify predictors of COVID-19 progression and survival.
The observed reduction was highest in CD8 T cells, which are important regulators of viral infection. T cells are broadly categorized into two groups, CD8 and CD4, based on their functions. CD4 T-cells are called “helper” cells and they mostly act as organizers by stimulating other immune cells and ensuring proper actions to restrict damage to surrounding tissue. This is the type of T cell that is deficient in patients with HIV. CD8 T-cell are sometimes called “killer” cells because they produce strong signals to infected cells to initiate programmed death of an infected cell.
Examination of the proteins present on cell membrane through the use of flow cytometry. The researchers identified three types of patients based on the presence of these proteins and a patient’s clinical outcome. Some patients had a profile of high activation in their CD4 T cells, with very high, possibly exhausted CD8 cells. A second group had high CD8 activity, but low CD4 activity. Lastly, there was a group of about 20% of patients that had no T cell response. Researchers don’t know the reason for these differences and additional research is necessary to fully understand the impact of these differences on each patient’s prognosis, survival and long term recovery.
Disparities or T cell diversity may have an impact on regulation of the immune response (CD4 T cells), myeloid cell recruitment (CD4 T cells), induction of cell death in infected cells (CD8 T cells) and antibody production (CD4 T cells). The finding of these differences between patients may help identify predictors of COVID-19 progression and survival. Our understanding of the differences between these groups may aid medical personnel in reducing mortality, increasing recovery and enhancing long-term prognosis of patients in each group.
Symptoms and differences in affected populations
A study of almost 45,000 people in China found that 81% of people get a mild form of the disease with fever, cough, sore throat and other symptoms of a cold. Fourteen percent of those infected get a severe form of the disease. These patients show difficulty breathing and fatigue. Finally, there are about 5% of patients that present a critical, life-threatning form of COVID-19 with respiratory and other organ failure.
While 25% of people infected with COVID-19 have co-morbidities, more than 60% of those in hospitals have co-morbidities. This disparity and suggests that co-morbidities exacerbate COVID-19. Another observed disparity comes from age when infected. Children (< 18 years of age) typically have milder symptoms that are limited to the upper respiratory tract and they rarely require hospitalization. In very rare circumstances a multisystem inflammatory syndrome has been reported in children of European descent.
Looking at populations with prior exposures to other viruses3
Another recent publication examined mounting evidence of COVID-19 patients with particular interest in their prior infections. Kadambari and colleagues suggest that previous infection with Cytomegalovirus (CMV), a common virus that infects large percentages of the population may explain some of the differences observed based on age. In people with a healthy immune system, the infection rarely causes problems.
The research group points out that as we get older we lose an important organizer of the immune response, T cells. As we age, our immune system becomes less effective and as a consequence older adults with a CMV infection may have complications. The CMV virus may suppress the immune system even further than the natural age-related decline.
In people previously infected and recovered from CMV the percentage of T-cells dedicated to fighting off virus is higher than in those that have never been exposed. The focus on fighting off the CMV leads to a lack of T cell diversity. This is compounded with a normal decline in T cell diversity as we age.
Because most T cells are singularly focused on fighting off CMV, the immune response to new antigens and pathogens is curtailed, leading to the cytokine storm observed in some patients. This T cell dysregulation, along with co-morbidities found in elderly patients, may explain the increased severity and mortality observed in the elderly.
Immune cells and development of severe COVID-19
Autopsies of COVID-19 victim’s reveals macrophages and mononuclear cells, some of the myeloid cells mentioned above, occupying the airspaces of the lung along with severe inflammation of the surrounding vascular tissue. Alveolar spaces, the tiny pockets of our lungs where gas exchange occurs are filled with liquid which starts to form a membrane indicating a condition called Acute Respiratory Distress Syndrome (ARDS).
In patients with ARDS the likelihood of dying is greatly increased and in survivors it causes lung tissue scaring and blood clots. It also predisposes survivors to infections and psychological symptoms. In some cases there is increased coagulation and complications with thrombosis, complicated with viral sepsis, which can lead to organ or multi-organ failure and death.
SARS-CoV2 transmission
Transmission mostly occurs through respiratory droplets generated when speaking with someone in close-quarters. Infected individuals may remain without symptoms for up to 11 days, although most people develop symptoms during the first week. Most infections are thought to be transmitted from infected individuals that show no symptoms. While spread from asymptomatic is likely very uncommon, although care should be taken since there is no way to distinguish asymptomatic individuals from pre-symptomatic individuals.
For these reasons people are asked to physically distance when outside of their homes. In situations where an individual may come in contact with another person, masks are suggested to reduce the emission or receipt of respiratory droplets. Outside of laboratories there is no evidence that the virus can remain in the air for a long time and cause infection through aerosols.
While the virus is able to survive on non-permeable surfaces, such as stainless steel or plastics for up to four days, there is likely minimal risk of contagion from surfaces. If you touch a surface outside your home, use soap and water (if available) to wash your hands before touching your face or alternatively use hand sanitizer. Avoid touching your face while out in public.
Treatment
More than 75% of those hospitalized with COVID-19 require oxygen. For some of these individuals positioning and muscle relaxants can help improve breathing. Other patients may require oxygenation and intubation to provide enough oxygen to survive.
Some patients may be treated with anti-viral drugs, although initial results indicate that hydroxychloroquine and lopinavir show no improvement over standard of care. Initial results of success by using convalescent plasma have also been tempered by reports of larger cohorts where no improvement is observed in severe and critical patients receiving this plasma.
Immunomodulatory drugs that reduce inflammatory proteins like IL-6 may be effective at controlling symptoms of unregulated inflammation and decreasing tissue and organ damage. Some studies suggest that immune damage to self could be controlled through the use of immune suppressant steroids such as dexamethasone and methylprednisolone to reduce the chance of ARDS. The benefits of using these medications must be weighed by medical personnel with access to a patients history and against the risk of secondary infections.
It is also important to remember that critically ill patients that recover continue to need medical assistance, in some cases for years. Survival from sepsis leads to long term physical disability, cognitive impairment and increased vulnerability to infection.
As we continue to learn more about SARS-CoV2 infection, opportunities to improve patient outcomes will increase. The shifting focus will be especially important to the recovery once the pandemic is under control and we are able to turn our attention to the needs of those recovering from the infection. The mental health toll of the strategies chosen to deal with the pandemic, the effects of the infection on people’s lungs and vascular tissue, along with possible cognitive impairment following COVID-19 will require novel strategies and will continue to impact our society for a long time.
References:
- Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J. & Prescott, H. C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. Jama 2019, 1–13 (2020).
- Mathew, D. et al. Deep immune profiling of COVID-19 patients reveals patient heterogeneity and distinct immunotypes with implications for therapeutic interventions. bioRxiv Prepr. Serv. Biol. 8511, 1–29 (2020).
- Kadambari, S., Klenerman, P. & Pollard, A. J. Why the elderly appear to be more severely affected by COVID-19: The potential role of immunosenescence and CMV. Rev. Med. Virol. 1–5 (2020) doi:10.1002/rmv.2144.
