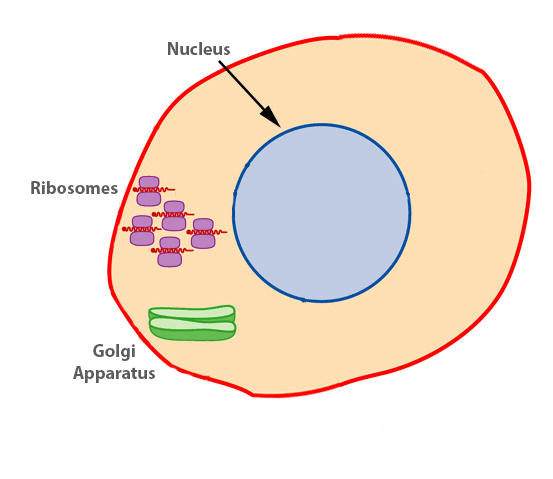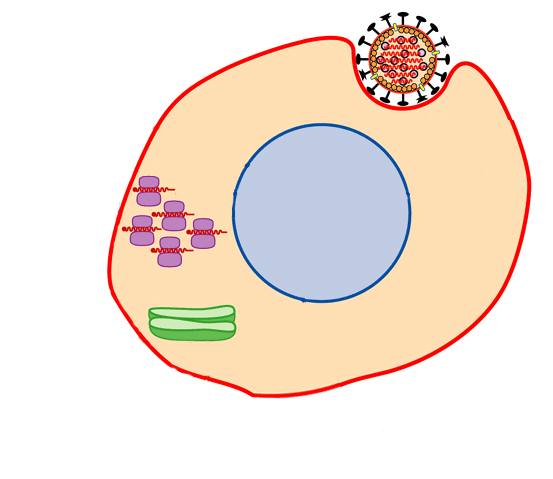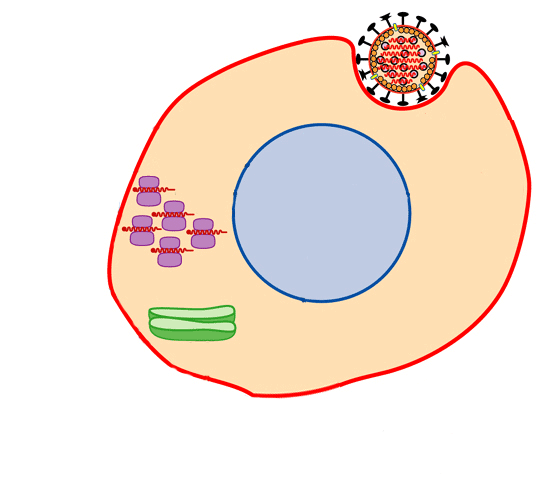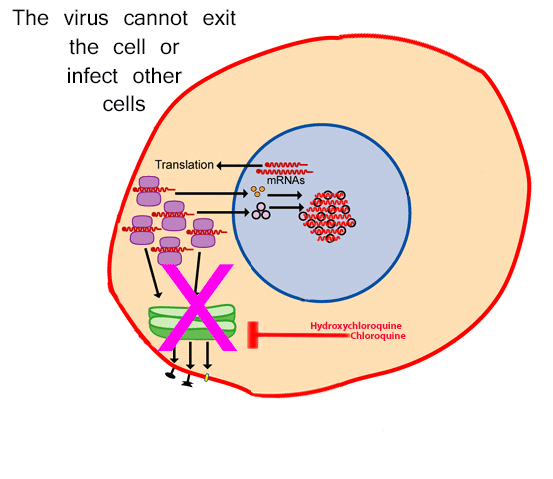
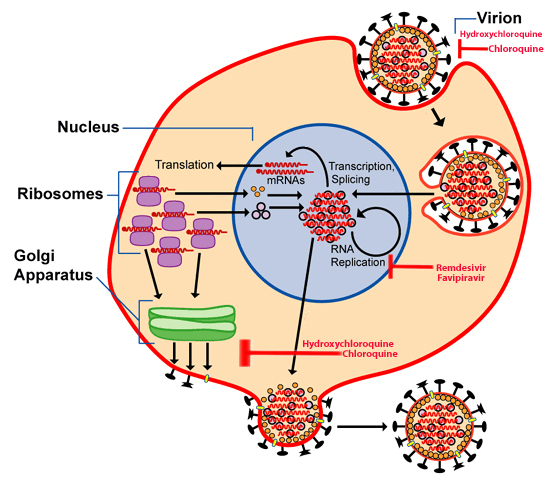
By Dr.Poonam Balaji
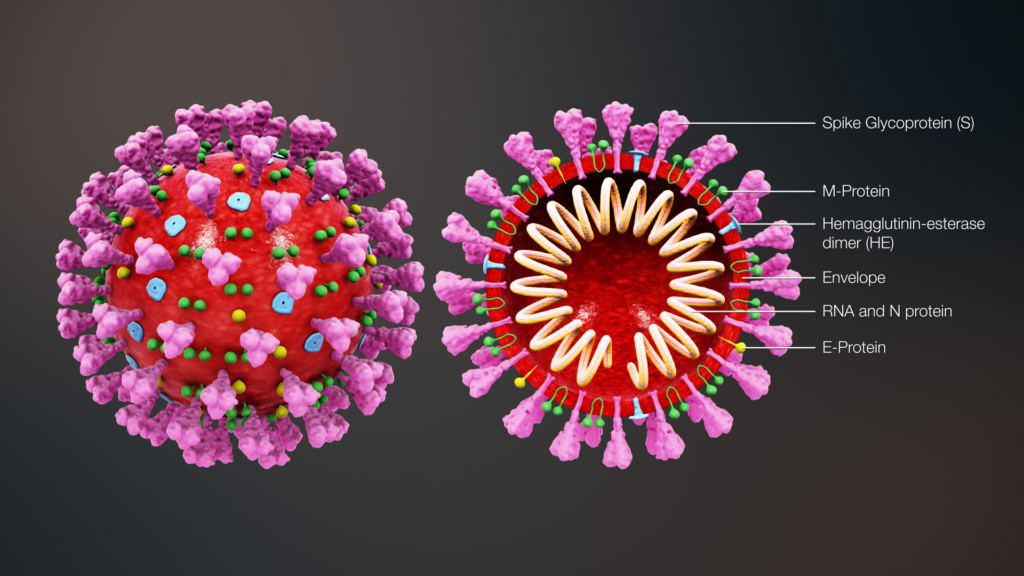
Vaccines are perhaps one of the greatest known inventions of science. We realize the significance of vaccination now more than ever as we desperately seek for a vaccination for the corona virus to stop the global pandemic.
Vaccines
The first vaccine was discovered by Edward Jenner in 1798, against small pox. Since the discovery of the small pox vaccine a number of vaccines have now become available against several deadly diseases such as polio, tuberculosis, measles, rubella, diphtheria etc. Thanks to vaccines some of the most deadly diseases such as polio1 and small pox2 are now almost eradicated from this planet.
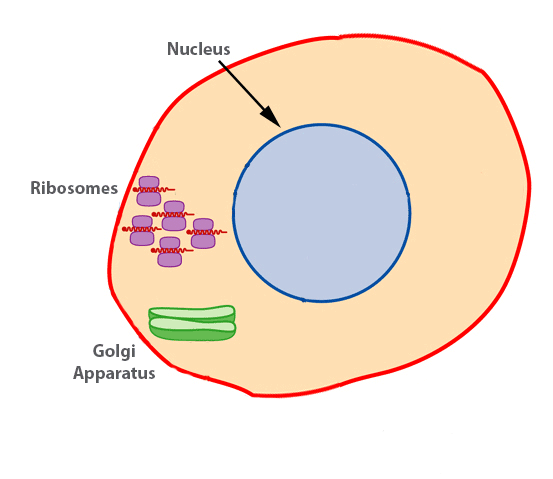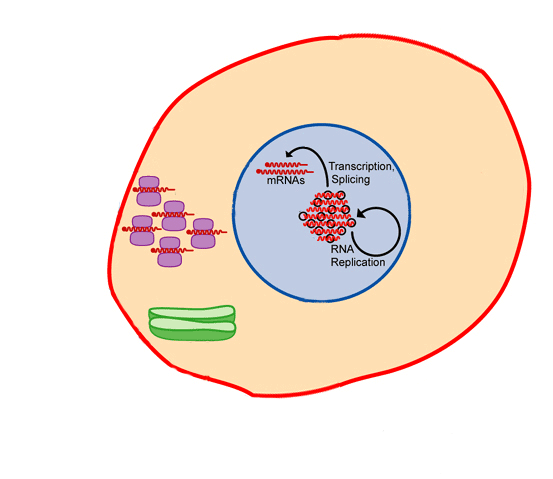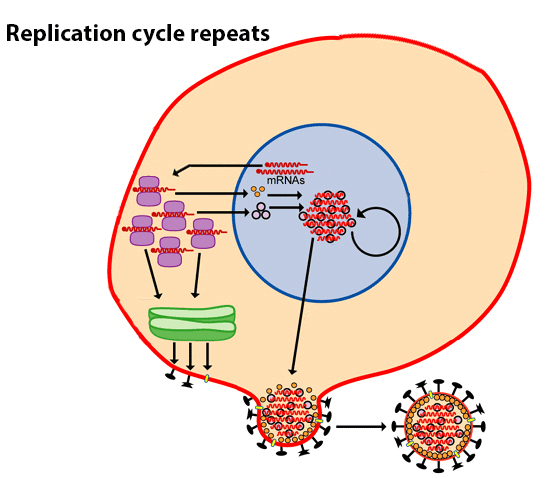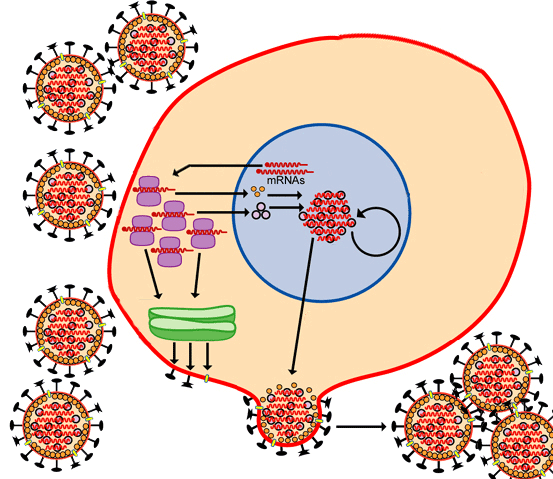
Vaccines train the human immune system to recognize and fight pathogens such as bacteria and viruses without exposing the body to the disease symptoms. Vaccines are made of inactivated/ dead or weakened form of the pathogens (antigens). They can’t cause an infection, but when the immune system is exposed to them, it still sees them as a foreign particle and produces antibodies in response.
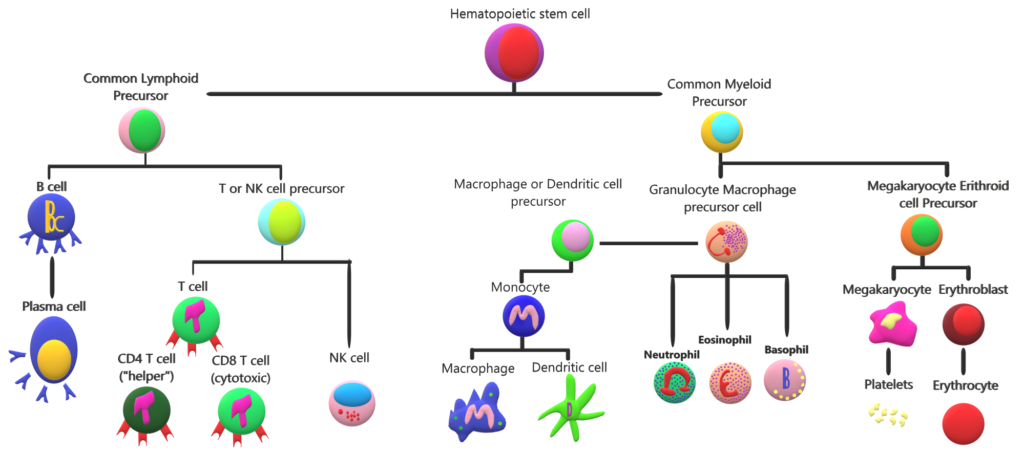
A primary goal of vaccination is generation of antibodies for a sustained period of time. Months after vaccination the antibody levels peak, followed by a decline to a plateau level which may then be maintained for decades with minimal decline. Research has shown that these plateau antibody levels are maintained by specialized non dividing cells in our immune system residing in the bone marrow called bone marrow plasma cells (BMPC)3. BMPC have the potential to survive indefinitely in both mice and humans4 while also continuously secreting antibodies. Further, total and antigen-specific serum antibody levels correlate closely with BMPC numbers in humans.
Why do you need to get a flu shot every year?
The best vaccines- measles, rubella, and diphtheria5– are the ones which offer 100% protection and last for life and you only need to take them once or twice (booster doses). Flu vaccines however are another story.
Research published in the journal Science by Rafi Ahmed and colleagues 6 from Emory University, Atlanta looked at BMPC to understand the sustainability problem of the flu shot. The antibody levels following vaccination depends on the number of BMPC7. The more the BMPC numbers greater the concentration of the serum antibody.
In case of the flu vaccine, antibody levels and protection conferred decline rapidly following vaccination, suggesting that the vaccine may fail to elicit BMPC, or that these BMPC fail to become long-lived. To investigate this the authors in this study examined the bone marrow and blood of 53 volunteers aged between 20 and 45 years old in the weeks and months before and after they received their flu shots. The unique aspect of this study is drawing the bone marrow from individuals which is a challenging and painful procedure that involves piercing the pelvic bone with a special needle.
28 days after flu vaccination the human influenza specific BMPC are generated and their numbers were significantly higher in these individuals then before the flu shot. But after 1 year, the new cells were virtually gone which explains why we would need to get a flu shot every year! According to the authors – “Several steps are required for a BMPC to become a long-lived plasma cell: the cell must reach the appropriate survival niche and successfully compete for space there, and the cell must undergo changes in gene expression and metabolism that promote longevity. Thus, our data suggests that most vaccine-induced BMPC fail at one or more of these steps.”
One of the suggestions made by the authors for improving the durability and efficacy of the flu shot by increasing the influenza specific BMPC numbers, is by utilizing adjuvants in the flu vaccine.
Adjuvants8 are ingredients (chemicals) added to vaccines to boost their immune response. In other words adjuvants make vaccines work better. Adjuvants have been safely used in vaccines for decades. In fact, the original flu vaccination did have an adjuvant.
A few modifications have been made to the flu vaccination over the past several decades. 1) The first influenza vaccines, developed in the 1940s, contained killed flu viruses mixed with a water-in-oil emulsion called incomplete Freund’s adjuvant. But the adjuvant was dropped in subsequent vaccines as it caused ulcers at the injection site. 2) Researchers have also stopped using the entire inactivate/ killed virus in the flu vaccine, replacing it with only the surface proteins from the virus to mitigate unwanted side effects. These flu vaccines are being used widely nowadays due to fewer side effects but at the cost of long term immunity.
Over the past decade a number of adjuvants9 are being deemed safe to be included in the flu vaccine and it would be interesting to see if they enhance the BMPC response of the flu shot. More detailed research is needed in this area. Until then the truth of the flu shot is that you need to keep getting one every year!
References:
- Soucheray, S. World Polio Day: Wild poliovirus type 3 declared eradicated. https://www.cidrap.umn.edu/news-perspective/2019/10/world-polio-day-wild-poliovirus-type-3-declared-eradicated.
- World Health Organization. Frequently asked questions and answers on smallpox. https://www.who.int/csr/disease/smallpox/faq/en/#:~:text=Smallpox was fatal in up,was in Somalia in 1977.
- Pioli, P. D. Plasma Cells, the Next Generation: Beyond Antibody Secretion. https://www.frontiersin.org/articles/10.3389/fimmu.2019.02768/full.
- Chernova, I. et al. Lasting Antibody Responses Are Mediated by a Combination of Newly Formed and Established Bone Marrow Plasma Cells Drawn from Clonally Distinct Precursors. J. Immunol. 193, 4971–4979 (2014).
- Cohen, J. How long do vaccines last? The surprising answers may help protect people longer. https://www.sciencemag.org/news/2019/04/how-long-do-vaccines-last-surprising-answers-may-help-protect-people-longer.
- Davis, C. W. et al. Influenza vaccine–induced human bone marrow plasma cells decline within a year after vaccination. Science (80-. ). 20, eaaz8432 (2020).
- Turesson, I. Distribution of Immunoglobulin‐containing Cells in Human Bone Marrow and Lymphoid Tissues. Acta Med. Scand. 199, 293–304 (1976).
- Control, C. for D. Adjuvants and Vaccines. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html#:~:text=What is an adjuvant and,adjuvants help vaccines work better.
- Tregoning, J. S., Russell, R. F. & Kinnear, E. Adjuvanted in fl uenza vaccines. 14, 550–564 (2018).